
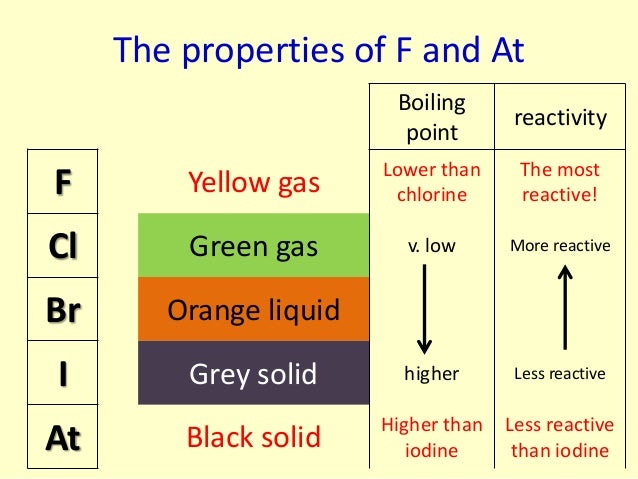
Has to be heated strongly and so does the iron wool.

The reaction is faster than that of iodine but slower than that of chlorine. Has to be warmed and the iron wool heated. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. Fluorine is the most reactive halogen, while astatine is the least reactive. Very few scientists handle fluorine because it is so dangerous. What is the trend in reactivity for the halogens in GCSE Chemistry The trend in reactivity for the halogens is that the reactivity decreases as you go down the group. You can see the trend in reactivity if you react the halogens with iron wool. Halogen have very high electronegativities They have seven valence electrons (one short of a stable octet) They are highly reactive, therefore toxics The. įluorine is the most reactive element of all in Group 7. As you move down the group, the amount of electron. 1.Trend in colour Halogens are coloured because of their low ionization energy.Electron excite to higher energy level by absorbing energy from visible region. Another trend going down group 7 is their reactivity.
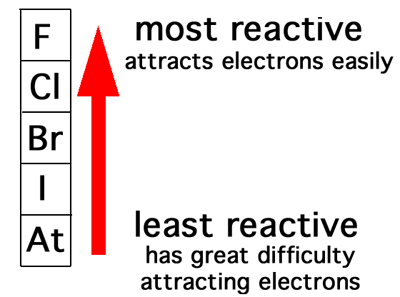
this is because atomic shielding increases going down the group, meaning out electron is further away from nucleus. Halogens react with sodium hydroxide in a disproportionation reaction. As you go down the group the trend in the atomic radius is that it increases the further down you go. Study with Quizlet and memorize flashcards containing terms like know the colours, physical states and trends in physical properties of these elements, how does displacement reactions involving halogens and halides provide evidence for the trend in reactivity in Group 7, explain the trend in reactivity in Group 7 in terms of electronic configurations and more. What is the trend in reactivity going down group 7 -going down group 7, reactivity decreases.
A brief chemistry of fluorine, bromine, chlorine, iodine and astatine is given which is then elaborated on in the subsequent pages - use the Group 7/17 halogen sub-index below. This is because group 7 elements react by gaining an electron. Reactivity decreases as you move down the group.
GROUP 7 REACTIVITY TREND HOW TO
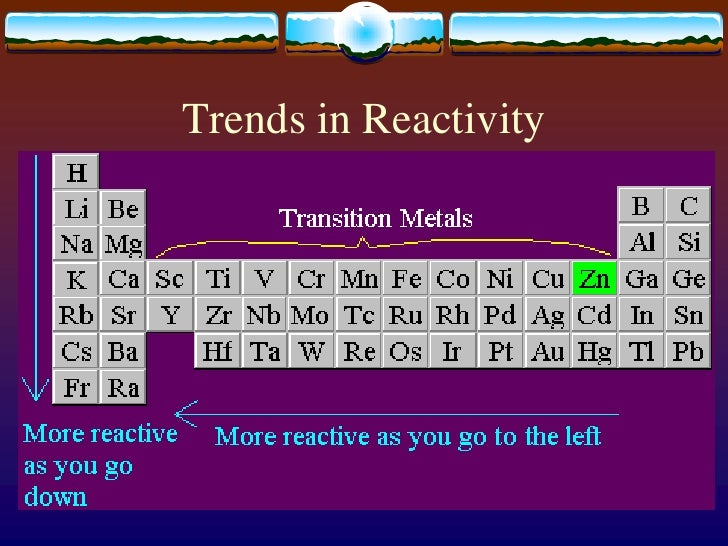
There is also a section on how to name halogen compounds. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic table. Group 7/17 halogen trends in properties are described, explained and discussed. Describes and explains the trend in oxidising ability of the Group 7 elements based on the reactions between one halogen and the ions of another one - for example, between Cl 2 and I - ions from salts like KI. The 2+ ions of the alkaline earth metals have a noble gas like electron configuration and are thus form colorless or white compounds (unless the anion is itself colored).The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group.


 0 kommentar(er)
0 kommentar(er)
